Introduction
Introduction
Objectively quantifying the mental state of individuals is a difficult task that lacks standardized, generalizable, and objective methods. For instance, law enforcement faces challenges in objectively determining if a driver is cannabis impaired, employers struggle with objectively assessing employees’ fitness for safety-critical duties, psychologists lack objective measures for therapy efficacy, psychiatrists face challenges in objectively evaluating drug efficacy, and Consumer Packaged Goods (CPG) brands lack a reliable means to objectively measure and prove the efficacy of their products. Implementation of existing solutions is often time consuming, non-scalable, and/or unreliable; manual EEG/brain-activity analysis and subjective assessments by health professionals are cited as non-scalable practices, and subjective surveys are subject to bias and contribute to unreliability. As such, there is a need for the provision of a cost-effective, readily-generalizable, scaleable, and standardized solution for accurately and objectively quantifying mental states.
In response to the current challenges in this space, Zentrela is developing generalizable predictive AI models to measure subjective psychoactive experiences, trained on an extensive electroencephalogram (EEG) recording dataset obtained through IRB-approved scientific trials totalling over one thousand study participants. Zentrela’s work is focused on objectively characterizing various mental states, such as energetic or relaxation, using a range of research tools including the EEG recordings, standardized psychological tests (e.g. BRUMS, BIG 5) EKG monitoring, and others tests like the Drug Effects Questionnaire (DEQ). Trials have been conducted at-scale since 2018, and consequently, has allowed procurement of an extensive database of EEG recordings accurately labeled with an individual’s psychological/mental-state characterizations and physiological measurements. Given its large size, the resulting EEG dataset presents a unique opportunity with which to train universal AI models that can quickly analyze new EEG data to objectively predict the mental state of an individual; a task typically completed “manually” by highly specialized health professionals, at a considerably high cost. Through this work, Zentrela maintains the goal of making neuroscience / EEG technology a commercially viable research tool for several markets outside hospitals and clinical setups, such as the labs of CPG companies, workplaces, etc. Zentrela’s AI-based EEG diagnostic tool, known as Cognalyzer®, aims to provide a standardized, objective and scalable solution to address the identified gaps. Zentrela’s proprietary and patent-pending method for training AI models has demonstrated high prediction accuracy, as exemplified by their first solution: Cognalyzer® AI for Cannabis’ Psychoactive Effects (Bosnyak et al., 2022; McDonald et al., 2021).
An immediate application of Zentrela’s portable EEG technology and Cognalyzer® AI solution has been for cannabis product testing. The EEG technology, first scientific trials, and the first AI diagnostic model (Cognalyzer® AI for Cannabis’ Psychoactive Effects) were funded by the Ontario Government (OCI and OBI) with an objective of adopting Zentrela’s portable EEG technology to help directly and objectively quantify cannabis-related psychoactivity, independent of conventional methods that may rely on detecting THC residuals in the bloodstream as a proxy for psychoactivity. The anticipated improvements consist of making Drug Recognition Evaluations more streamlined and objective in assessing whether an individual is experiencing acute cannabis’ psychoactive effects or not. This development transforms the Cognalyzer® into a commercially applicable tool with a long-term perspective, anticipating legislative changes necessary for authorizing Zentrela’s portable EEG technology for law enforcement use.
As an extension to its original intent, legal cannabis producers and brands have partnered with Zentrela to utilize the Cognalyzer® AI for Cannabis’ Psychoactive Effects to evaluate the specific psychoactive effects of their products in detail. This type of accurate and objective product effect analysis is designed to determine the efficacy of products to create targeted effects for specific consumer profiles. Further, the Cognalyzer® AI has been helping them to refine product formulations, remain in compliance with the Cannabis Act regulations, and to launch education programs about product effects for cannabis retailers and consumers as a way of differentiating their products and brands. As such, Zentrela’s Cognalyzer® AI has emerged as the standard in the global cannabis market for objectively quantifying the psychoactive effects of legal cannabis products.
Building on this success, Zentrela introduces a new universal AI model: the Cognalyzer® AI for Relaxation. This model is designed to quantify and predict the mental state of relaxation, defined here as where an individual experiences a sense of sleepiness, calmness, reduced mental activity, and decreased levels of stress and anxiety associated with the processes of falling asleep with ease. Application of the Cognalyzer® AI for Relaxation further aims to assess how relaxation levels are influenced by internal (wellness/health product consumption, meditation) and external factors (listening to music, music therapy). This paper describes the objectivity of the EEG, psychological, and physiological data collection methods, and the subsequent prediction accuracy of the Cognalyzer® AI for Relaxation. Results from controlled trials characterizing the relaxation effects of wellness/energy products are also presented to illustrate the Cognalyzer® AI for Relaxation accuracy and effectiveness.
Zentrela Research Framework
Zentrela Research Framework
General EEG Study Framework
Since 2018, Zentrela has completed numerous IRB-approved studies under Health Canada’s Observational research framework to develop an accurate and objective evaluation of mental-state, mood, and psychoactive effects. These studies employ the use of EEG recordings collected using Zentrela’s end-to-end proprietary Electroencephalography (EEG) system, which aims to capture the distinct electrical neural signatures associated with mental states (e.g. energetic, fatigued etc.).
An individual’s electrical brain activity is periodically recorded through EEG during a specified trial period, and the complementary physiological and physiological tests are administered in parallel. Depending on the objective of a given study, the individual may ingest a substance (e.g. coffee) or engage in activities (e.g. listening to music) that can induce psychoactivity and shifts in mental states, wherein the resulting EEG recordings and test results thus characterize the expected psychoactive effects. These studies have enabled the accumulation of a substantial EEG database, incorporating many tens of thousands of EEG recordings from over one thousand research participants, and comprises the training and testing data utilized in development of Zentrela’s novel AI solution; the Cognalyzer®. Additional detailed information regarding the specific EEG research protocols is provided in the associated documents for the Z-CUSIC-01, Z-CUSIC-02, and Z-CUSIC-03 studies, as well as the peer-reviewed studies completed by Bosnyak et al., (2022) and McDonald et al., (2021), for reference.
EEG recordings are obtained from patients with Zentrela’s proprietary headset, fitted with 8 non-invasive electrodes strategically placed on right and left sides of the frontal, temporatal, parietal and occipital lobes to collect brain activity from the main regions of the brain. The headset collects and streams data via Bluetooth to a nearby laptop. Data is recorded continuously throughout a given trial using an 8-channel EEG system with a 250-Hz sampling rate and 24-bit resolution. Each recording is meticulously screened for artifacts post-hoc, and unreliable data segments are rejected in real-time. For each measurement, data collection continues until a sufficient amount of reliable, artifact-free segments of EEG recordings are obtained. The relevant psychological and physiological evaluations (i.e. BRUMS, DEQ) are administered concurrently with the EEG measurements. Several measurements are made before (control) and throughout the expected period of mental state alteration or psychoactivity. The resulting data comprises a coarse time-series of detailed brain activity recordings (EEG), mental state/psychoactivity characterizations (BRUMS, DEQ), and physiological measurements (EKG).
EEG Data Analysis and Labeling
Certain induced and implicit mental states and traits, including fear (Zhou et al., 2021), craving (Garrison et al., 2023), reward (Speer et al., 2023), learning (Kincses et al., 2023 ), and cognition (Sripada et al., 2020), among others, are known to produce distinct neural signatures in aspects such as power spectral density, cross power spectral density, coherence, and RMS power in EEG recordings. Following the EEG recordings and associated data-screening, neurological signatures are extracted from the EEG using Zentrela’s proprietary Cognalyzer® algorithm. The Cognalyzer® scientifically characterizes and labels each EEG recording in regard to the presence/absence and magnitude of certain mental states and emotional traits. Characterization and labeling is completed in accordance with established neural signatures, a proprietary library of neural signatures, and the corroborating psychological and physiological data.
Sensitivity and specificity of the Cognalyzer® algorithm for cannabis-induced psychoactive mental state predictions have been evaluated and validated by external CRO trials, as detailed in Bosnyak et al., (2022) and McDonald et al., (2021). Zentrela’s unique methodology allows for objective conceptualization of mental states from EEG that has a proven high level of correspondence with subjective evaluations on an individual level. The Cognalyzer® algorithm evolves through continuous refinement, integrating data from numerous individuals to enhance its ability to objectively estimate the psychoactive effect level. Additional labeled data from each individual contributes to refining and validating the algorithm’s performance. Detailed psychographic data collection allows the creation of more discriminators for further enhancing classification. The original labeled EEG data ensures that enhancements can always be reapplied, providing ongoing insights. As such, this methodology provides a generalizable framework to predict and investigate a wider range of mental states and their relation to cannabis, or other substances with perceived psychoactive effects.
Cognalyzer® AI Relaxation Development
Zentrela has developed a novel AI-based predictive tool designed to identify whether an individual is in a mental state characterized by relaxation. This tool, named the Cognalyzer® AI for Relaxation, was trained and tested using Zentrela’s extensive, proprietary EEG database. Consequently, the Cognalyzer® AI for Relaxation makes predictions based on the EEG recording segments of an individual, whether, and to what extent, a patient is experiencing a mental state associated with relaxation (i.e. sleepiness, calmness, restful etc.). As such, this presents the opportunity to test and evaluate psychoactive substances (e.g. cannabis) in regard to their ability to induce said relaxation effects.
In regards to labeling individual observations with relaxation effect of interest, EEG recording segments were characterized based on established and proprietary neural signatures, and the results of the complementary/non-EEG tools (e.g. BRUMs). The resulting characterizations describe an individual’s mental state in terms of relaxation (calm, de-stressed, hypnagogic) or non-relaxation (energized, alert, hypnopompic), and the magnitude of such. An individual identified as having sufficient indicators of relaxation is coded as “1” (termed “Relaxed”), whereas those with a lack of indicators is coded “0” (termed “Non Relaxed”).
Consistent with its predecessors (e.g. Cognalyzer® AI for Cannabis’ Psychoactive Effects), the Cognalyzer® AI for Relaxation works in a two-stage process; first processing the EEG data and extracting the relevant features and signatures, and subsequently predicting the mental state of the associated individual. EEG data processing involves signal conversion and extraction of thousands of instances of several EEG features related to this mental state (e.g. frequency range 8-12Hz). Model selection and associated parameter tuning was based on cross-validation on the test set comprising 4155 EEG recordings, wherein the best-performing models were adopted with predictions aggregated via majority-vote. To test performance in practical settings, this solution is then applied to new EEG data collected under controlled scenarios, both before and after the consumption of a product or exposure to external factors that may influence relaxation levels, such as listening to music.
Cognalyzer® AI Relaxation Effect Study
Cognalyzer® AI Relaxation Effect Study
Cognalyzer® AI Relaxation Study Methodology
Zentrela has completed an applied research study to investigate the relaxation-related effects of a cannabis product using the Cognalyzer® AI for Relaxation. Similar practical application studies are routinely completed by Zentrela and offer a means to validate and assess the accuracy of the Cognalyzer® AI for Relaxation (Version AZRM6) model, amongst other models, following initial model building and training from the EEG database. These studies serve to improve the effectiveness and commercial applicability of the Cognalyzer® AI solutions, such that established and published objective EEG results can be replicated and provided at-scale to clients. Further, these studies are used to characterize the impact of wellness solutions on the relaxation state of consumers, with the objective of helping wellness companies (CPG brands, therapies, etc.) to objectively prove the efficacy (i.e. onset time and strength/effectiveness) of their solutions to inform and help customers achieve their desired state of relaxation or wakefulness. Zentrela’s practical application study for the Cognalyzer® AI for Relaxation is conducted in accordance with Zentrela’s established framework, as described in the above sections, and is designed for the purpose of objectively quantifying the efficacy of wellness CPG products (like cannabis products, natural health products, energy drinks or any other product that is formulated to relax or energy consumers) by conducting scientific / controlled human trials (Observational, Controlled, Clinical, or Therapeutic). Zentrela
In this study, 29 research participants consumed a beverage infused with 10mg of THC, 10mg of CBD and magnesium (i.e. the wellness product). To investigate the impacts of the wellness product, the non-invasive portable EEG device and desktop application were used to record study subject’s EEG data from 8 different regions of the brain (right and left sides of the frontal, temporal, parietal and occipital lobes). The standardized process of collecting raw EEG data, (called “EEG Test”) consists of around 2-minute-long recordings depending on the quality and integrity of the EEG data that is being recorded, where internal and external noise is ignored. Two EEG Tests were administered before product consumption to characterize the baseline “mental state“ (the initial relaxation level of consumers). Following consumption of the wellness product, EEG Tests were administered 5, 10, 15, 20, 25, 30, 45, 90, 120 minutes post-consumption. The complementary psychological and physiological tests were administered periodically throughout the EEG monitoring period.
The top existing Cognalyzer® AI Relaxation model specification (AZMR6) from the test EEG dataset was selected for practical testing on the novel EEG data from the control trials. The AZMR6 model was applied to the EEG data obtained from the 29 research participants to characterize and generate an aggregate relaxation effect curve spanning the period of induced mental-state change; upon gathering sufficient EEG data, the Cognalyzer® AI Relaxation analyzes each EEG test segment individually, subsequently characterizing the baseline Relaxation Effect Level (%) from the pre-consumption recordings, and any associated trends in Relaxation Effect Level from the post-consumption recordings. Relaxation Effect Level was interpolated to a one-minute temporal resolution using a cubic spline method. Data from each participant group were aggregated thereafter for analyses. One and two-standard deviation confidence intervals of Relaxation Effect Levels at each time-point were produced, wherein a significant trend or deviation from the baseline level could be identified. Further, any identified trends were corroborated with the complementary psychological and physiological test data. Namely, a curve of the concurrent aggregate BRUMs test results, on aggregate, is generated to compare and validate model results.
Cognalyzer® AI Relaxation Study Results
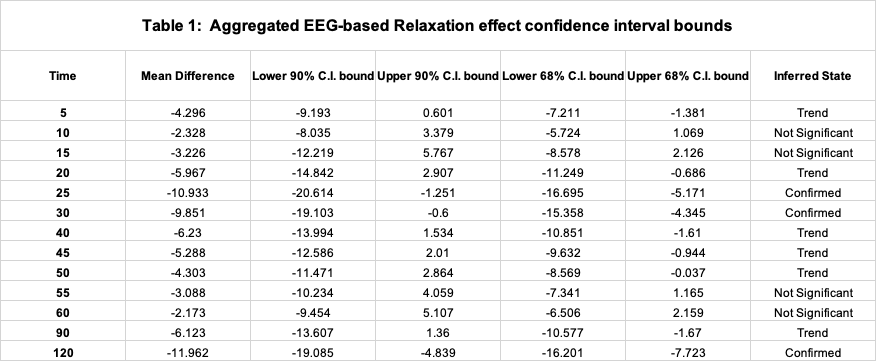
A time-series plot of the aggregated EEG data and corroboratory BRUMs psychological test data is presented in Figure 1, below. The mean difference between baseline relaxation and post-consumption relaxation, along with associated 1 and 2 standard deviation confidence interval bounds, is provided in Table 1 for each recorded timestep. Regarding the inferred (i.e. predicted) state of an individual, on aggregate, a level of ‘Trend’ is associated with a predicted relaxation effect 1 standard deviation in excess of baseline conditions and a ‘Confirmed’ level indicates an exceedance of two standard deviations.
On aggregate, there is a general observable trend of an initial spike in relaxation, peaking at around 25-30 minutes after product consumption. A significant relaxation effect is predicted to occur during this ‘peaking’ period. This is followed by a gradual offset of the effect, until roughly 60 elapsed minutes, and a gradual minor increase in the effect thereafter. This trend generally aligns with the respective BRUMs Vigor subscale psychological tests, which peaks at 25-30 minutes, but does not show the gradual offset of the effect thereafter.
The top performing model specifications each individually achieved an accuracy, sensitivity, and specificity all ranging between 75% and 80% when cross-validated against the large existing test EEG dataset (4155 EEG recordings), which is labeled with the associated BRUMs results consistent with the present study. Based on previous applications of these models on novel data from Zentrela’s human trials, aggregation of model predictions by majority-voting can be expected to increase this accuracy by up to 10 percentage points. Similar improvements are expected with further calibration and tuning of the AZMR6 model specification upon additional procurement of additional trial data.
Research Framework
Research Framework
| Research Organization: | Zentrela, Inc. |
|---|---|
Research Framework: | Observational |
Consumer Attestation: | Fully informed consent form (ICF) |
Principal Investigators: | Dr. Dan Bosnyak, Israel Gasperin |
Authors: | John Tweedie, Upmanyu Sharma, Israel Gasperin Haaz, Dr. Dan Bosnyak |
Research Facility Location: | Zentrela Lab, 231 Main St W, Hamilton, ON, Canada. |
Conclusion
Zentrela’s research and technology addresses the challenge of objectively quantifying mental states by developing cost-effective, scalable, and standardized AI-based solutions. Zentrela’s proprietary methods have demonstrated high levels of accuracy for mental state prediction, and have consequently become a widely adopted commercially applicable tool for wellness product testing and formulation refinement. Numerous IRB-approved studies have been conducted by Zentrela since 2018 to objectively evaluate mental states, moods, and psychoactive effects by employing a proprietary EEG headset. The resulting EEG recordings, along with parallel physiological and psychological tests, contribute to a substantial database used to develop Zentrela’s Cognalyzer® AI solutions, including Cognalyzer® AI for Relaxation. The culmination of these efforts positions Zentrela’s Cognalyzer® AI as a valuable tool in objectively quantifying mental states, contributing to advancements in neuroscience applications beyond clinical settings.
Zentrela’s latest AI-based solution, the Cognalyzer® AI for Relaxation, was developed, trained, and tested in accordance with Zentrela’s established IRB-approved research framework. A practical application study using the Cognalyzer® AI for Relaxation was conducted to investigate the relaxation effects of a wellness product, aiming to objectively quantify its efficacy and to assess the utility of the Cognalyzer® itself. When study subjects were exposed to a wellness solution formulated for relaxation (a cannabis-infused beverage with 10mg THC and 10mg CBD) the participants’ average relaxation level significantly increased above baseline. Further, the study demonstrated a general alignment between the Cognalyzer® AI for Relaxation and the observed, unbiased psychological tests and mental state characterizations (i.e. BRUMs), on aggregate. This alignment is further illustrated by the 75 – 80% accuracy, sensitivity, and specificity achieved by applying the Cognalyzer® AI for Relaxation on the existing EEG test dataset. The study results suggest that the Cognalyzer® AI for Relaxation is capable of identifying and quantifying changes in a relaxation mental state following ingestion of a substance or participation in an action that is intended to induce a relaxation effect, such as cannabis-infused products, music therapy, Natural Health Products, Medicine, etc.. Further research and model-prediction optimization is required and will be conducted to refine/tune the Cognalyzer® AI for Relaxation solution.
References
Bosnyak, D., McDonald, A. C., Gasperin Haaz, I., Qi, W., Crowley, D. C., Guthrie, N., & Evans, M. (2022). Use of a Novel EEG-Based Objective Test, the Cognalyzer®, in Quantifying the Strength and Determining the Action Time of Cannabis Psychoactive Effects and Factors that May Influence Them Within an Observational Study Framework. Neurology and Therapy, 11(1), 51–72. https://doi.org/10.1007/s40120-021-00293-w
Garrison, K. A., Sinha, R., Potenza, M. N., Gao, S., Liang, Q., Lacadie, C., & Scheinost, D. (2023). Transdiagnostic Connectome-Based Prediction of Craving. American Journal of Psychiatry, 180(6), 445–453. https://doi.org/10.1176/appi.ajp.21121207
Kincses, B., Forkmann, K., Schlitt, F., Pawlik, R., Schmidt, K., Timmann, D., Elsenbruch, S., Wiech, K., Bingel, U., & Spisák, T. (2023). RCPL preprint: An externally validated resting-state brain connectivity signature of pain-related learning. https://doi.org/10.31219/osf.io/utkbv
McDonald, A. C., Gasperin Haaz, I., Qi, W., Crowley, D. C., Guthrie, N., Evans, M., & Bosnyak, D. (2021). Sensitivity, Specificity and Accuracy of a Novel EEG-Based Objective Test, the Cognalyzer®, in Detecting Cannabis Psychoactive Effects. Advances in Therapy, 38(5), 2513–2531. https://doi.org/10.1007/s12325-021-01718-6
Speer, S. P. H., Keysers, C., Barrios, J. C., Teurlings, C. J. S., Smidts, A., Boksem, M. A. S., Wager, T. D., & Gazzola, V. (2023). A multivariate brain signature for reward. NeuroImage, 271, 119990. https://doi.org/10.1016/j.neuroimage.2023.119990
Sripada, C., Rutherford, S., Angstadt, M., Thompson, W. K., Luciana, M., Weigard, A., Hyde, L. H., & Heitzeg, M. (2020). Prediction of neurocognition in youth from resting state fMRI. Molecular Psychiatry, 25(12), 3413–3421. https://doi.org/10.1038/s41380-019-0481-6
Zhou, F., Zhao, W., Qi, Z., Geng, Y., Yao, S., Kendrick, K. M., Wager, T. D., & Becker, B. (2021). A distributed fMRI-based signature for the subjective experience of fear. Nature Communications, 12(1). Scopus. https://doi.org/10.1038/s41467-021-26977-3
Copyright, Disclaimers and Terms of Use
Copyright © 2022 Zentrela Inc. All rights reserved.
This website, source code, databases, functionality, software, website designs, audio, video, text, photographs, graphics, and data on the website and the relevant Zentrela trademarks, service marks, and logos contained therein (the “Material”) are owned or controlled by or licensed to Zentrela Inc. a corporation having its principal place of business at 231 Main St. W., Hamilton, Ontario, L8P 1J4, Canada (“Zentrela”).
The Material is protected by copyright and trademarks laws and other various other intellectual property rights and unfair competition laws of Canada, the United States, foreign jurisdictions and international conventions. All other trade names, trademarks, service marks and other product or service names and logos on or in the Material are the proprietary trademarks of their respective owners and are protected by applicable trademark and copyright laws.
Use of this website is subject to Terms of Use.

