Introduction
Introduction
On August 18th, 2018, the Canadian government made the decision to legalize cannabis consumption for recreational purposes. It has had far-reaching implications across multiple sectors and stakeholders of this industry. The government is taxing this new market to generate a new income for the Canadian economy, licensed businesses (producers and retailers) can sell cannabis legally and Canadians have safe access to cannabis as another recreational alternative besides drinking alcohol, taking natural products, or practicing a recreational activity like yoga to relax. In the study “Understanding the Motivations for Recreational Marijuana Use Among Canadians”, researchers from the University of Calgary further explain how people use recreational cannabis to enhance their leisure activities and manage the challenges and demands of our daily lives, like relaxing at the end of the day.
After 5 years of this recreational cannabis era we’ve witnessed new and innovative ways of cultivating cannabis and developing unique product formulations in an effort to deliver the product experience consumers seek.
In the case of the development of product formulations like gummies, capsules, chocolates, tablets, cartridges for vaporizers, etc, it is well known that the way these products are formulated significantly impact their efficacy to create intended benefits, like fast onset, strong and long lasting effects, or the opposite, slow and mellow onset but long lasting effects. For example, our previous EEG-based research objectively and quantitatively establishes how product formats and cannabinoid delivery methods significantly affect the onset time and potency of a cannabis psychoactive effect (EEG-based studies CUSIC-3, CUSIC-4, 2021-2023). We have also generated evidence regarding how the method of extracting cannabinoids from the plant can also impact the potency of a product (EEG-based study CUSIC-6, 2023). In addition to all the product development factors already described, the cannabinoid composition, the amount of each cannabinoid, and the combination of additional ingredients like terpenes, black tea (caffeine), etc. create unique formulations and determine how effective a product will be at creating the intended effects.
The main objective of this study was to objectively measure the efficacy of Kinloch’s Serene™ CBD Green Apple Gummy Drop (the “Investigational Product”) to increase the relaxation state of consumers. Each unit (one gummy) of the Investigational Product is infused with 50 mg of CBD and terpenes: Beta-Caryophyllene, Beta-Pinene, Linalool, Myrcene, Trans-Beta-Caryophyllene (Source: OCS.ca, 2024-02-29) with the strategic objective of enhancing its efficacy at increasing relaxation levels for consumers.
Previous research has shown that CBD has the potential to stimulate the state of mind of consumers to make them feel more relaxed or sleepy. Also, a recent study shows that terpenes play a key role to enhance the effects of cannabinoids.
This crossover EEG-based study, CUSIC-25, is the first of its kind designed to objectively quantify how effective a CBD-only product formulation truly is at increasing the relaxation state of consumers. To accomplish this research objective, the same group of study participants consumed the Investigational Product and a Comparator product (that also contains 50mg of CBD but does not include terpenes) in 2 different visits to the Zentrela lab on the same day of the week, at the same time of the day, and under the same controlled conditions. Then their EEG was collected and analyzed through standardized and objective protocols.
The results of this study provide objective and quantifiable evidence regarding the relaxation effectiveness of both products, highlighting substantial discrepancies in efficacy despite their equal CBD content.
NOTE 1: A point worth noting is that the novelty of this research not only relies on the use of EEG technology but how the EEG analysis is conducted. Previous EEG-related studies have shown that EEG has the potential to capture EEG feature changes caused by CBD. However, these studies have been conducted in clinical setups only (using clinical EEG systems and specialists to clean EEG data, analyze it, and interpret it). This traditional way of EEG analysis makes it slow and extremely expensive for organizations that do not have clinical setups like R&D labs of large CPG companies for product efficacy testing. Zentrela’s pioneering approach for EEG analysis is driven by scientifically proven Machine Learning models trained on massive EEG datasets recorded during controlled trials to accurately capture and measure specific mental states, like relaxation.
Zentrela’s first AI solution is the Cognalyzer® AI for Cannabis’ Psychoactive Effect (See Paper 1 and Paper 2). For this study, the AI solution used to analyze the EEG of study subjects is the Cognalyzer® AI for Relaxation, which has been trained to quantify the mental state of relaxation, defined as: being sleepy, calm, not mentally active, less stressed and anxious associated with the processes of falling asleep (See Paper 3).
NOTE 2: It’s important to highlight that this trial is not about determining the therapeutic benefits of relaxation but simply quantifying how effective the Investigational Product is at altering this mental state.
Methodology
Methodology
To accurately measure the potential of the Investigational Product and the Comparator to create relaxation effects, a cross-over study was conducted in Zentrela’s lab where the following human and environmental factors were kept under control in order to minimize their effect on the results:
Human variability: the same group of 15 participants consumed both products in the same environment.
Consumption amount: consumers bought and consumed 1 unit (containing 50mg CBD) of each product. On Visit 1 they consumed the Comparator and on Visit 2 they consumed the Investigational Product.
Consumption time: on both Visits, study subjects consumed their products at 7pm ET.
Room environment: For the first 60 minutes post product consumption, participants stayed in the data collection room, auditory stimulus was minimized and there was limited interaction between subjects. 60 minutes after EEG Tests, study subjects took breaks in the lounge rooms of the lab where they were mentally active, socializing, listening to music, watching videos, taking calls, or having food.
Room temperature: ~75F
Food / Drinks consumption: Participants reported not having consumed cannabis, alcohol nor any other drug or relaxation-related stimulant like tea, coffee or energy drinks in the last 12 hours before their participation in this study). During their visits, they drank water and consumed snacks like chips/popcorn.
Fifteen (15) consumers participated in this study. Ten (10) participants were female, and five (5) were male.
Zentrela’s portable non-invasive 8-channel EEG technology was used to record the electrical activity of the main regions of the brain (the right and left sides of the frontal, temporal, parietal, and occipital lobes).
Through Zentrela’s standardized EEG data collection process, multiple ~2-min-long EEG recordings (called “EEG Tests”) were taken before and after the consumption of the Investigational Product and a similar version (the “Comparator”). Two EEG Tests were taken before product consumption to characterize the initial relaxation state of research participants, and multiple EEG Tests were performed after the consumption of the product, at intervals 10-15 min, 25-35 min, 60-70 min, 120-130 min, and 170-180 min.
Zentrela’s AI solution for standardized and objective EEG-based relaxation analysis, called Cognalyzer® AI for Relaxation, version AZRM6, was used to process every EEG Test from this study group and return a relaxation level (RL) per EEG Test. Further explanation about what Relaxation means and the scientific validation regarding the objectively, accuracy, sensitivity, specificity this AI-based EEG analysis can be found here (www.zentrela.com/publication/cusic-24).
The cubic spline interpolation method was used to accurately estimate the RL of every participant’s experience at the same time intervals: 0, 5, 10, 15, 20, 30, 35, 40, 45, 50, 55, 60, 90, 120, 180 min post product consumption. The Relaxation Effect Levels (REL) of a product were determined by measuring the change of RL of every study subject after product consumption from their respective baseline RL (REL = RL after product consumption – RL baseline before product consumption).
The RELs were aggregated by product to quantify their respective Relaxation Effect Curves/Profiles (REC), which consists of the RLs over the time period of the study .
The RELs were also sub-grouped by the reported gender of every participant, with the purpose of studying the potential change of the Relaxation Effect Curve based on the “female” and “male” subgroups.
Along with the calculation of RELs, statistical analysis was carried out to determine the statistical significance (90% and 68%) of RELs at each time interval.
Study Results
Study Results
The time evolution of the relaxation effect curves (REC) of the study group after consuming both products are shown in Figures 1 and 2. Figure 3 shows both product RECs.
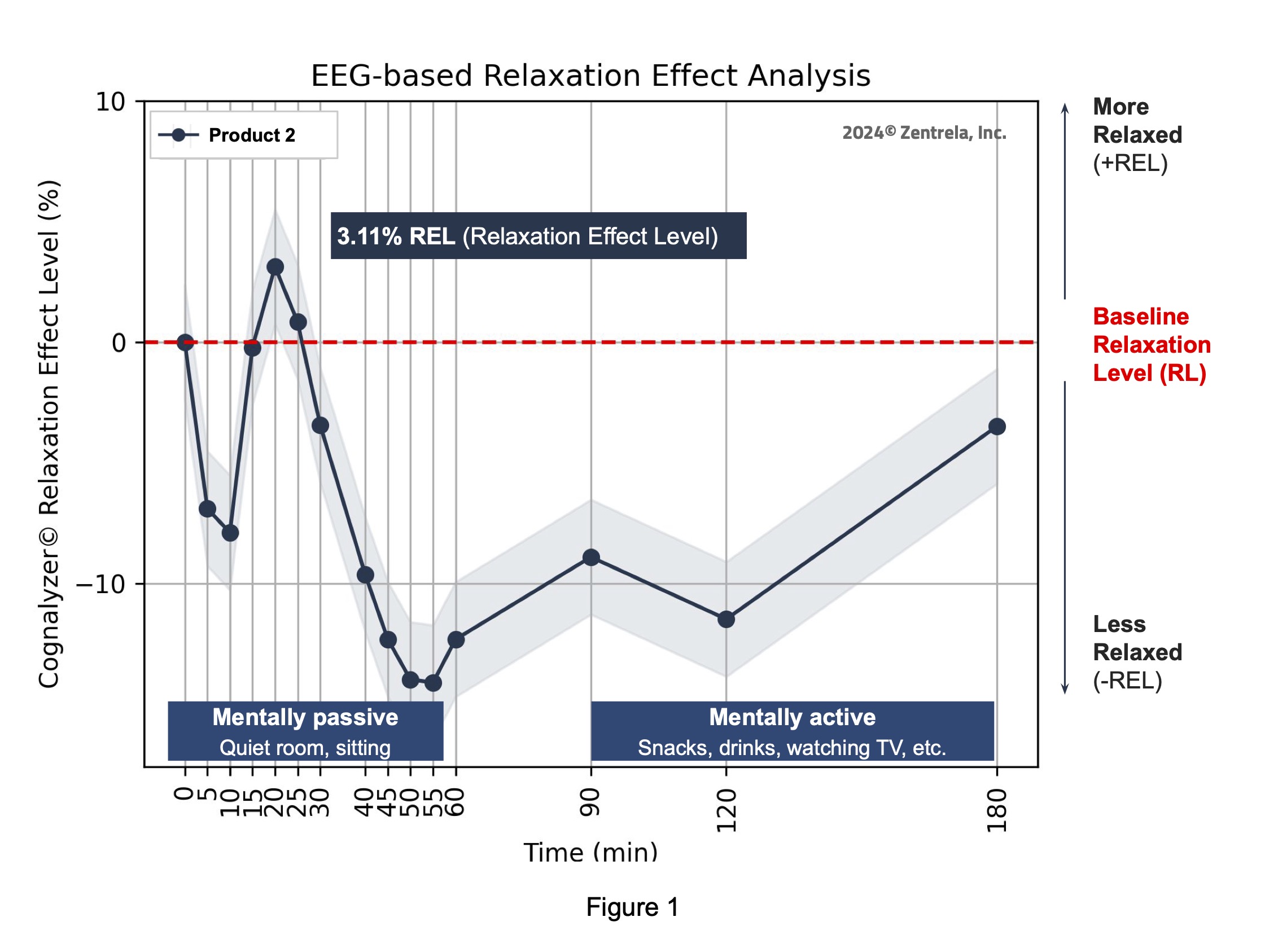
Comparator’s relaxation effect analysis
The maximum peak of increase in relaxation that the Comparator product created was 3.11% REL on the Cognalyzer® Relaxation Effect Scale, which occurred around 20 minutes post product consumption. However, Table 2 shows this increase of relaxation was not >90% statistically significant. Table 2 also shows, with 90% confidence, that in fact the relaxation state of the study group decreased around 45-60 minutes after consuming the Comparator product.
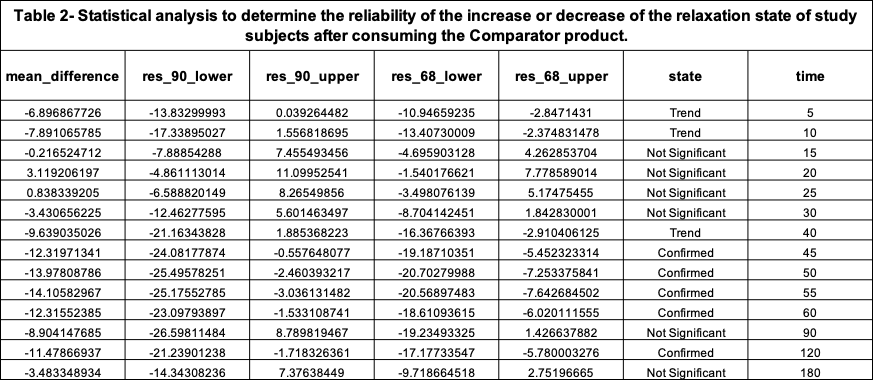
Table 2 shows that if an REL (mean_difference) is above 0 and the lower bound of its 90% confidence interval (res_90_lower) is also greater than 0, then with 90% confidence it is confirmed that the product increased the relaxation state of the study group after intaking the Comparator product. However, if an REL’s lower bound of its 90% confidence interval is equal or below to 0 but its lower bound of its 68% confidence interval (res_68_lower) is above 0 then the state (reliability) of the REL value is just a “Trend to increase relaxation”.
If an REL is below 0 and the upper bound of its 90% confidence interval (res_90_upper) is also below 0, then with 90% confidence it is Confirmed that the Comparator product decreased the relaxation state of the study group after intake. However, if an REL’s upper bound of its 90% confidence interval is equal or below to 0 but the upper bound of its 68% confidence interval is below 0 then the state (reliability) of the REL value is just a “Trend to decrease relaxation”.
The state “Not significant” means that it’s not reliable to determine if an REL increased or decreased the relaxation state of the study group after product consumption.
The same statistical methodology, explained above, was performed to analyze the RELs of the Investigational Product which is displayed below.
Serene™ CBD Product’s relaxation effect analysis
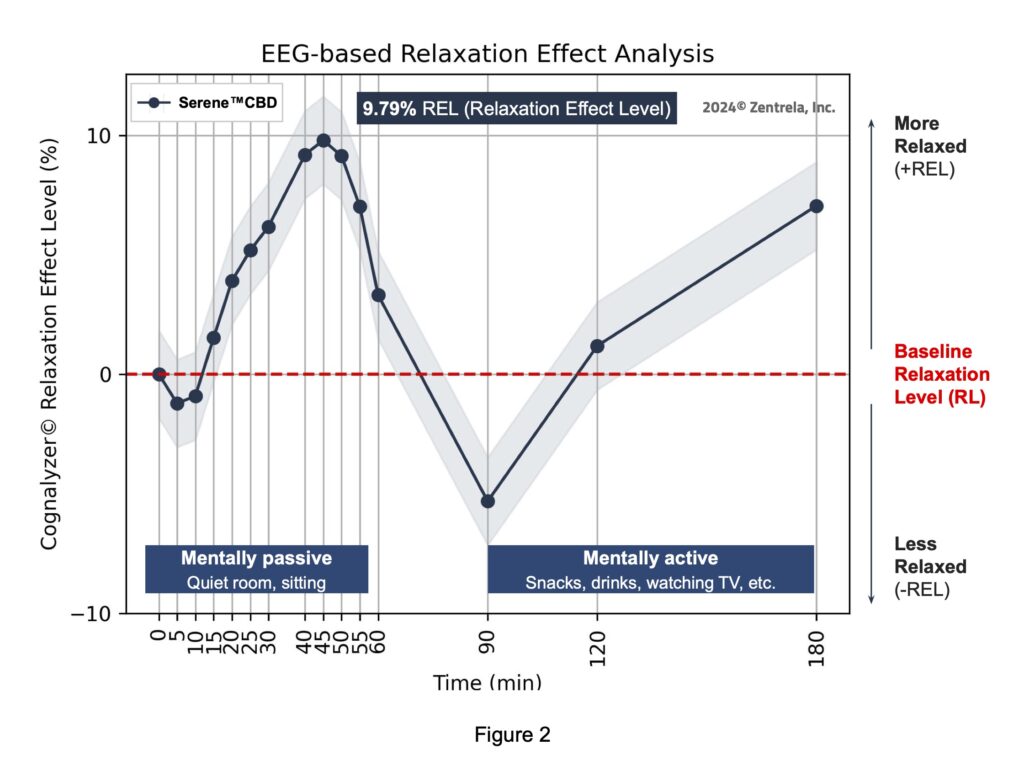
According to this scale, the maximum peak of increase of relaxation that study subjects experienced after consuming 1 unit of the Investigational Product was 9.79% REL which occurred around 45 minutes post consumption.
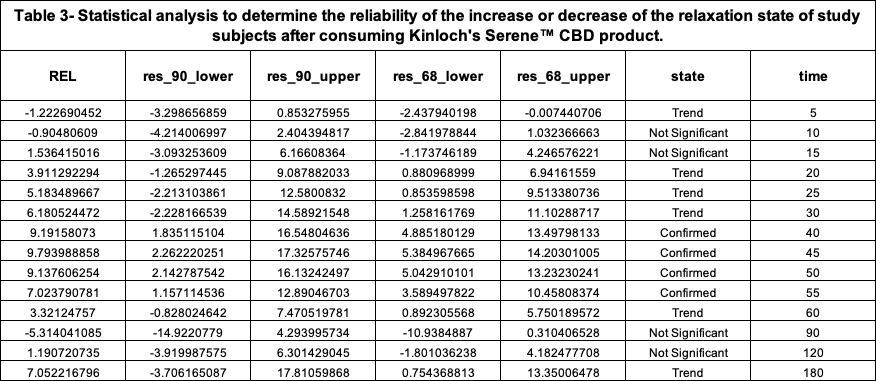
The following plot, Figure 3, along with the statistical comparison analysis represented in Table 4, elucidates and graphically depicts the increased relaxation state induced by both products among the participants of the study.
Benchmark analysis of both product’s efficacy to increase the relaxation state of study subjects
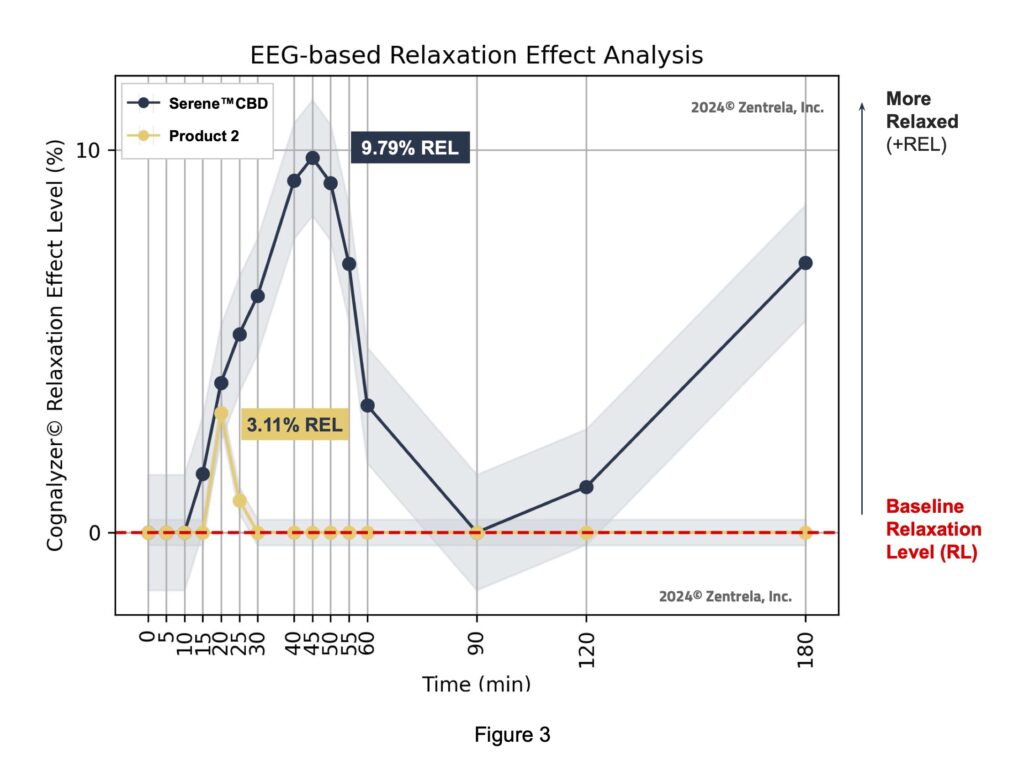
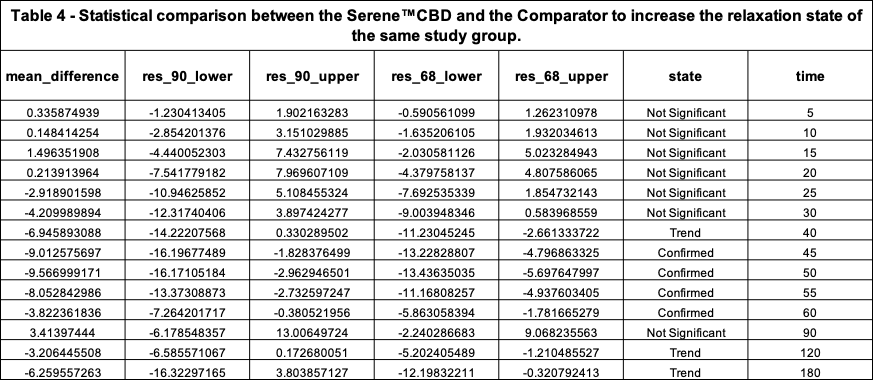
Table 4 describes the results of the comparative analysis between the Investigational Product and the Comparator (Product 2). The difference in mean relaxation effect levels is provided (“mean_difference”) at each time interval, along with their respective 68% and 90% confidence levels.
At a 90% confidence level, we can be certain that the true difference between the product’s RELs is between res_90_lower and res_90_upper. If res_90_upper (the upper confidence level bound) is negative, we can be certain (at a 90% confidence level) that the Serene™ CBD Green Apple Gummy Drop product (Investigational Product) is creating an REL greater than the Comparator. This is the state “Confirmed”.
The performed comparative analysis shown in Figure 3 and Table 4 suggests, with 90% confidence, that the Serene™ CBD Green Apply Gummy Drop product was ~3X more effective than the Comparator at their respective moments of Peak REL to increase the relaxation state of the study group.
Benchmark analysis of both product's relaxation efficacy for the female sub-group
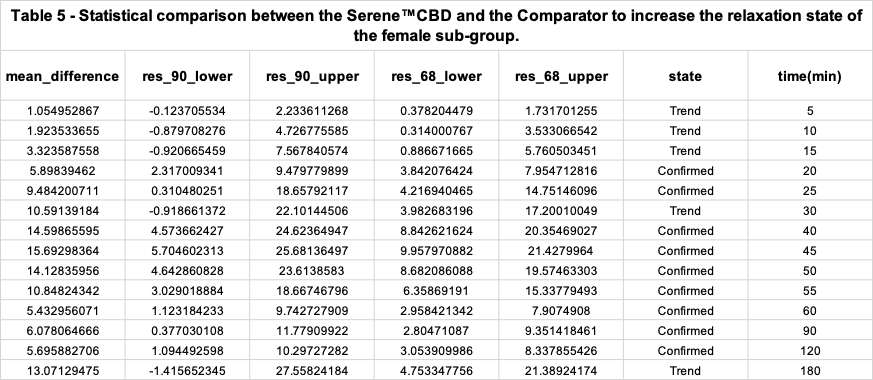
Dose efficacy analysis based on the gender of research participants
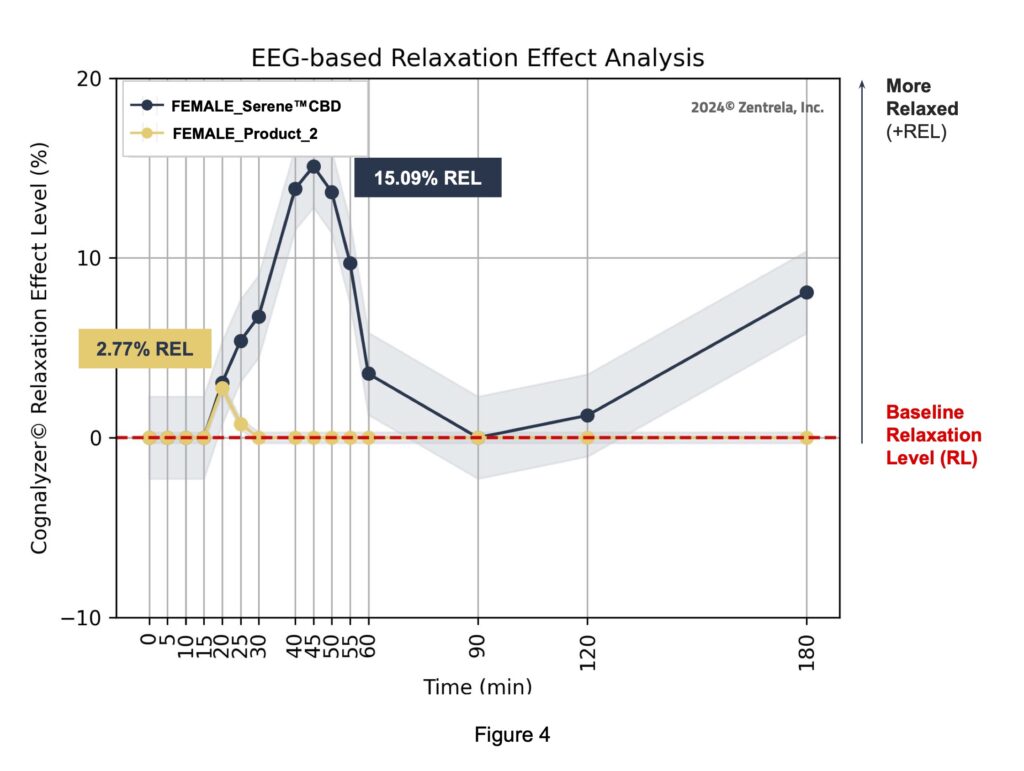
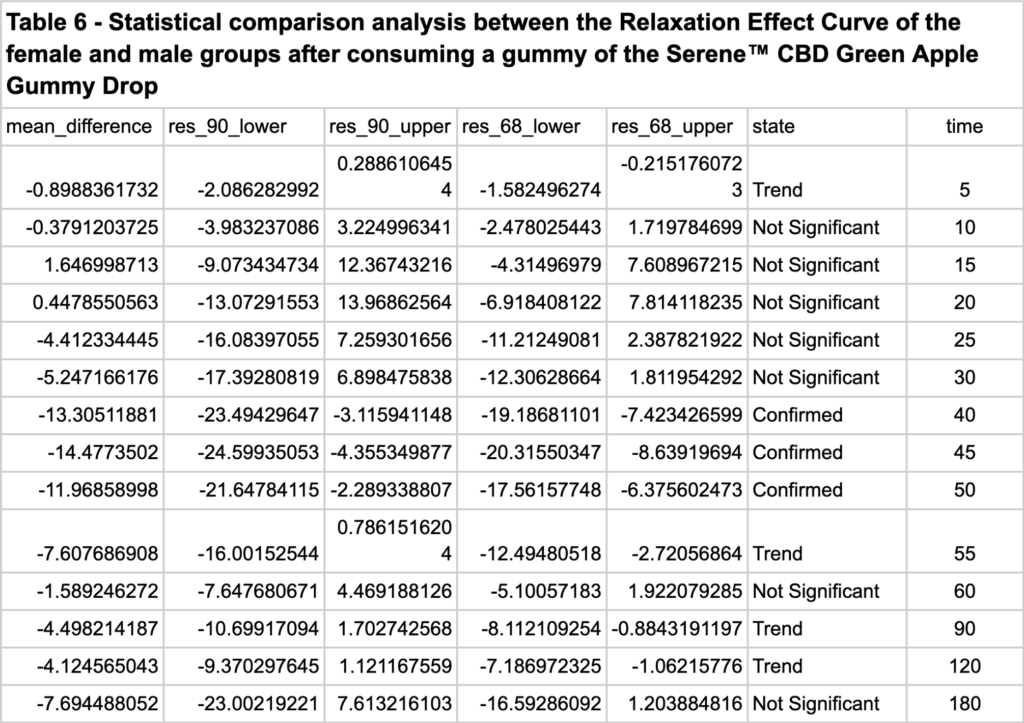
The Relaxation Effect Curve (REC) of the male group was also generated and compared to the REC of the female group.
Figure 5 shows that 1 gummy of the Serene™ CBD Green Apple Gummy Drop increased the relaxation mental state of the female group by 15.09% and 5.6% in the male group in the Cognalyzer® REL scale.
Research Framework
Research Framework
| Research Organization: | Zentrela, Inc. |
|---|---|
Scientific Research Model: | Z-CUSIC-02 Observational study of non-therapeutic cannabis effects |
IRB Approval: | Approved by the IRB, Advarra, Inc. |
Consumer Attestation: | Fully informed consent form (ICF) |
Principal Investigator: | Dr. Dan Bosnyak |
Authors: | I. Canchola, I. Gasperin, D. Bosnyak. |
Study Size: | 15 study subjects, 2 visits, 1 visit per product |
Research Tools: | Cognalyzer® AI-driven EEG analytics |
Research Facility Location: | CL1 231 Main St W, Hamilton, ON, CANADA |
Conclusion
The evidence from this study suggests that:
1. The Kinloch Serene™ CBD Green Apple Gummy Drop was ~3X more effective at increasing the relaxation state of the study group than the Comparator. This objective and standardized relaxation analysis shows that even though 2 products contain the same amount of CBD, they may have drastically different effectiveness at relaxing consumers. This difference in relaxation efficacy may be due to the product format, drug delivery method, the unique composition of the Kinloch Serene™ CBD Green Apple Gummy Drop, that not only includes 50mg of CBD but also different terpenes, or a combination of the above.
2. One unit of the Kinloch Serene™ CBD Green Apple Gummy Drop, taken around 7pm under the same controlled circumstances, may significantly increase the relaxation state of consumers around 40 minutes post consumption. However, the “trend” statistical analysis suggests that the onset time of the relaxation effect could be as early as 20 minutes post product consumption. Additional research is required to confirm this “trend”.
3. In regard to the female subgroup, the relaxation efficacy of the Kinloch product was 5X greater than the Comparator.
4. This evidence also indicates that the female subgroup was even more sensitive to the Kinloch Serene™ CBD Green Apple Gummy Drop than the entire group of study participants. Further research is required to perform this type of human variability analysis. The potential opportunity of this type of analysis is to find the accurate consumption amount of a specific product for a specific consumer type to guarantee delivery of the intended effect.
5. It is also worth mentioning that these results provide additional evidence about the importance of environmental factors to maximize the potential relaxation effect of products. Listening to music, watching videos, and socializing/chatting keep us mentally active. Further research will be performed to measure the impact of these environmental variables to our relaxation state.
6. Regarding consumption quantities, a single unit (gummy) of the Serene™ CBD Green Apple Gummy Drop demonstrated significantly greater efficacy in the female group. This observation suggests that the male cohort may require a higher dosage—potentially two gummies—to achieve a comparable level of relaxation as observed with a single gummy in the female cohort. It is important to consider that the observed difference in relaxation between these two groups may not be solely attributable to the gender of the study subjects, but rather to other physiological factors associated with their gender. For instance, males may exhibit a higher BMI. Further research is necessary to explore the variability in product/dose efficacy due to these human factors. Finding the right consumption amount of a product for different target audiences is an opportunity to help them achieve the desired effects and benefits.
Recommendations for the global cannabis industry
1. The key insight from this study is the contrasting efficacy of two products that contain the same amount of CBD (50mg per unit) to create an intended effect.
The concern of this finding is that CBD, THC and other ingredient properties are the only information consumers have to make “informed” purchase and consumption decisions. However, the objective and quantifiable evidence from this study clearly shows how unreliable it is asking consumers to make decisions based solely on the ingredient information of a product. The quality of the ingredients to be extracted, the drug delivery system used to infuse them in the product as well as other factors, all together play a critical role in determining the efficacy of a product to create an intended effect.
2. Cannabis consumers should become aware of this critical situation. It is a social responsibility (and mandate for retailers) to help consumers make informed purchase and consumption decisions. Consumer and budtender education programs should be developed for this purpose and it is imperative that this education happens at an individual product level (rather than generalizing statements like “THC intoxicates you”) to provide consumers with the most accurate information.
3. Zentrela’s portable EEG solution and AI-based EEG analysis have been designed to address this unmet global market need for objective and standardized product effect testing, that requires to be conducted relatively rapidly and cost-effectively.
4. In addition, objective human effect testing should be conducted to determine the viability of commercializing cannabis CPG products BEFORE they’re introduced into the market. Zentrela is already in the process of streamlining a viable (fast, affordable, objective, and regulatory-compliant) process of incorporating this new transformational type of product testing as a core operation and best practice in the business of developing legal cannabis to guarantee the Return Of Investing in commercializing these new products. This is becoming possible thanks to the pioneering regulatory work that Health Canada is performing through the creation of the Category 3 Non Therapeutic Research on Cannabis (NTRC) framework.
5. Truly innovative licensed cannabis companies should take the initiative to adopt this viable product testing methodology as a new core activity in the business of producing and selling cannabis products legally. Many pioneering cannabis labs have already started to use Zentrela’s portable EEG device for their own internal studies at an exponentially lower cost and faster pace than contracting a research organization.
Copyright, Disclaimers and Terms of Use
Copyright © 2022 Zentrela Inc. All rights reserved.
This website, source code, databases, functionality, software, website designs, audio, video, text, photographs, graphics, and data on the website and the relevant Zentrela trademarks, service marks, and logos contained therein (the “Material”) are owned or controlled by or licensed to Zentrela Inc. a corporation having its principal place of business at 231 Main St. W., Hamilton, Ontario, L8P 1J4, Canada (“Zentrela”).
The Material is protected by copyright and trademarks laws and other various other intellectual property rights and unfair competition laws of Canada, the United States, foreign jurisdictions and international conventions. All other trade names, trademarks, service marks and other product or service names and logos on or in the Material are the proprietary trademarks of their respective owners and are protected by applicable trademark and copyright laws.
Use of this website is subject to Terms of Use.